It is a clear colorless liquid with a sweet fruity smell.
Vinyl acetate monomer structure.
A series of vinyl acetate vv10 binders with different vv10 content was formulated in a 60 pvc matte paint.
Vinyl acetate monomer is stored in mild steel storage tanks and or new or reconditioned steel drums and can be transported by bulk vessels or tank trucks.
Enantioselective catalysts that have been applied to.
There are three different types of eva copolymer which differ in the vinyl acetate va content and the way the materials are used.
Search results for vinyl acetate at sigma aldrich.
Vinyl acetate monomer vam is a high volume building block chemical used in the manufacture of polyvinyl acetate or vinyl acetate copolymers.
Vinyl acetate is the acetate ester of vinyl alcohol.
Vinyl acetate and allyl cyanide are two of the representative substrates for asymmetric hydroformylation because alfa acetoxy propanals are versatile chiral building blocks that can be converted into amine alcohols oxazolines and imidazoles and the hydroformylation product of allyl cyanide is an intermediate for beta amino acid.
It is very flammable and may be ignited by heat sparks or flames.
Hence such systems may fail during wet cleaning or scrubbing.
Vinyl acetate is an industrial chemical that is produced in large amounts in the united states.
This is much less the case when the polymer contains a sufficient amount of hydrophobic monomer.
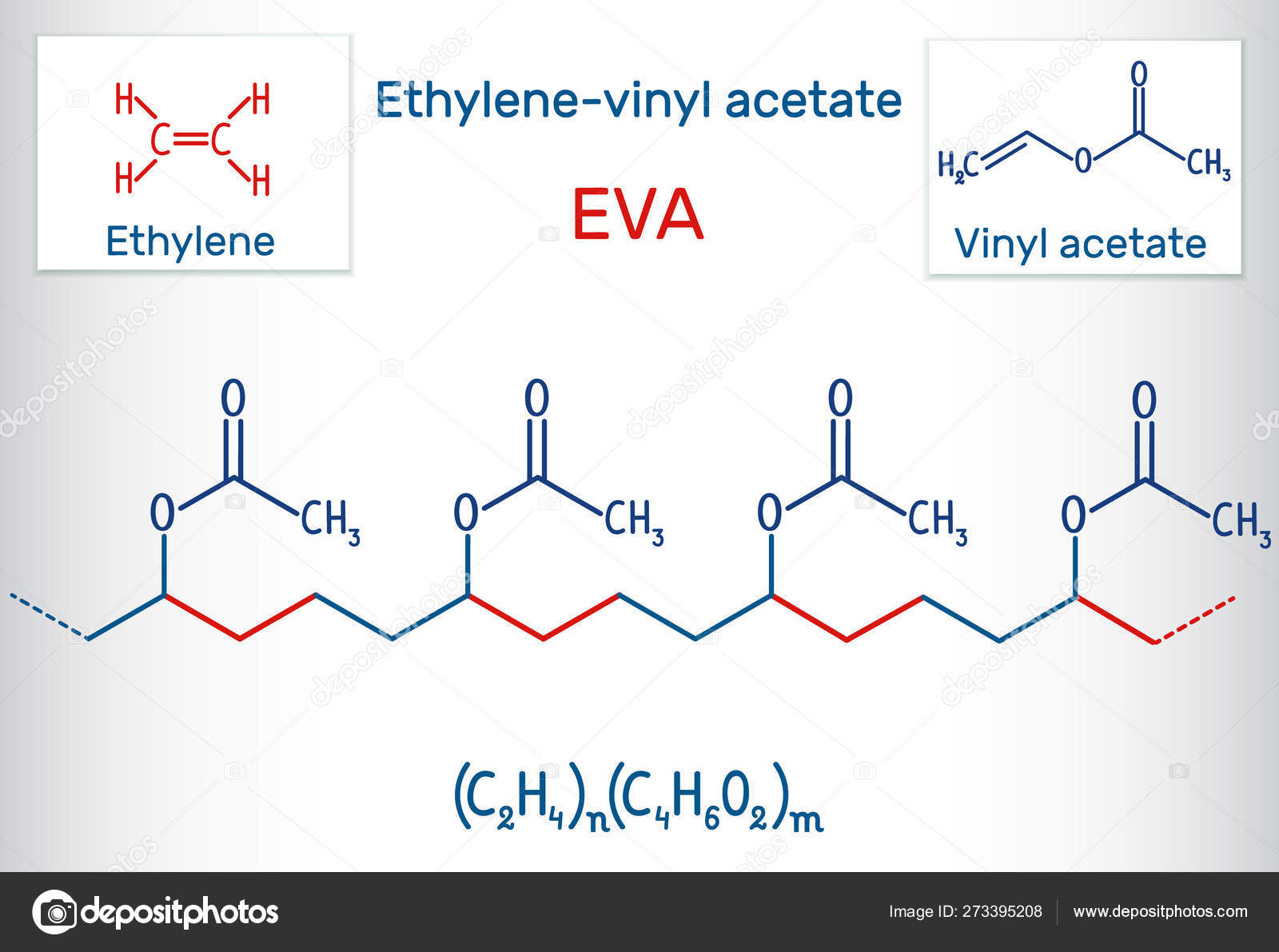
Ethylene vinyl acetate eva also known as poly ethylene vinyl acetate peva is the copolymer of ethylene and vinyl acetate the weight percent of vinyl acetate usually varies from 10 to 40 with the remainder being ethylene.
Vinyl acetate monomer vam is a key intermediate used in the making of a number of polymers and resins for adhesives coatings paints films textiles and other end products.
Please select more than one item to compare.
When handled properly vam can be stored transported and otherwise managed safely.
Since vinyl alcohol is highly unstable with respect to acetaldehyde the preparation of vinyl acetate is more complex than the synthesis of other acetate esters.
Vinyl acetate is used to make other industrial chemicals.
These polymers are the base for many industrial and consumer products as well as for other polymers.
Vinyl acetate ch 2 cho 2 cch 3 is prepared from ethylene by reaction with oxygen and acetic acid over a palladium catalyst under the action of free radical initiators vinyl acetate monomers single unit molecules can be linked into long branched polymers large multiple unit molecules in which the structure of the vinyl acetate repeating units is.
The largest derivative is polyvinyl acetate pva which is mainly used in adhesives as it has good adhesion properties to a number of substrates including paper wood.
Vv10 performs better in this respect than other co monomers for vinyl acetate.