The correlation analyses of a series of experiments using the phenoxyimine ligands ticl 4 initiating systems indicated that the substituents on the n aryl.
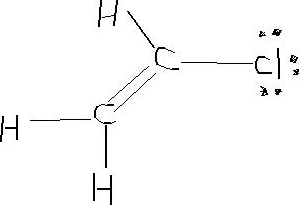
Vinyl chloride resonance structure.
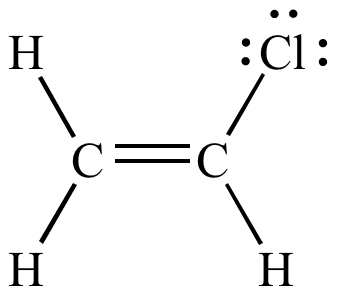
Cl have lone paire so it donate to next carbon atom.
Click here to get an answer to your question c cl bond in ch2 ch cl vinyl chloride is stabilised in the same way as in.
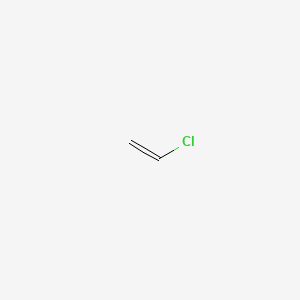
This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride.
Chemical bonding and molecular structure.
Vinyl chloride is an organochloride with the formula h2c chcl that is also called vinyl chloride monomer or chloroethene.
Playlist is at web site.
Resonance structures of a molecule do not have.
In terms of its molecular structure it consists of a vinyl group linked to a nitrile it is an important monomer for the manufacture of useful plastics such as polyacrylonitrile.
Vcm is among the top twenty largest petrochemicals in world production.
About 13 billion kilograms are produced annually.
The electron density between the two carbon atoms is less than that expected for an isolated double bond.
Vinyl chloride is primarily used to make polyvinyl chloride to manufacture plastics.
There are 3 resonating structure of vinyl chloride.
Resonance is due to non binding electrons to positive charged atom.
Second will be more stable than third because negative charge is more stable on more electronegative atom.
It is a colorless volatile liquid although commercial samples can be yellow due to impurities.
In resonance language it is said that the carbon chlorine bond in vinyl chloride or in chlorobenzene has some double bond character.
It has a pungent odor of garlic or onions.
Vinyl chloride is a chlorinated hydrocarbon occurring as a colorless highly flammable gas with a mild sweet odor that may emit toxic fumes of carbon dioxide carbon monoxide hydrogen chloride and phosgene when heated to decomposition.
So the least stable structure will be third resonating structure.
Acrylonitrile is an organic compound with the formula ch 2 chcn.
Trimethyl vinyl ammonium chloride.
The first structure will be most stable because it is neutral.